Solanum jamesii

| Common Names | 4 Corners potato, Four Corners potato, Colorado wild potato, Arizona wild potato, Sego |
| Code | jam |
| Synonyms | |
| Clade | 1 |
| Series | Pinnatisecta |
| Ploidy | Diploid (2x) |
| EBN | 1 |
| Tuberization Photoperiod | Long Day |
| Self-compatibility | No |
| Nuclear Genome | B |
| Cytoplasmic Genome | Unknown |
| Citation | Torrey: Ann. Lyceum Nat. Hist. New York 2(5): 227. 1828. |
Description
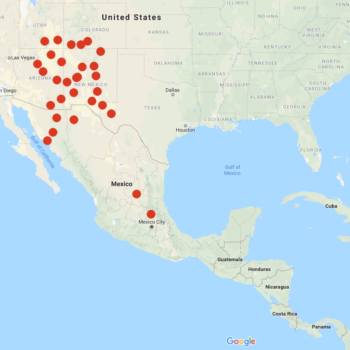
Solanum jamesii, sometimes known as the “Four Corners potato,” is a wild potato of the United States and northern Mexico, with a couple of separate populations in central Mexico. It is the only wild species with a distribution primarily in the United States, where it is found mostly in New Mexico and Arizona, but also reaches slightly into Colorado, Utah, and Texas at elevations above 5000 feet. Plants are small, reaching about 6 to 15 inches in the wild, but sometimes taller under cultivation. Foliage ranges in color from light green to blue-green. Flowers are white, stellate, with yellow anthers. Berries are most commonly light green with light blue speckles or vertical stripes, but can range in color from pale green to dark purple/black.
The specific epithet honors Edwin James, a naturalist who first formally collected and described the species. While there is no completely standardized pronunciation for scientific names, the most common way to pronounce this species is probably so-LAY-num JAME-zee-eye.
S. jamesii tubers are small, rarely reaching an inch in diameter and perhaps twice that for elite selections under cultivation. Plants grown at Cultivariable have produced an average of 102 tubers, with a average total yield of 66 grams and an average tuber weight of 0.67g, which is 678 tubers per pound or 1492 tubers per kilogram. The highest average tuber weight that I have seen is 3.45 grams and the largest tuber I have seen was 20.4 grams. The tubers are usually white to tan, turning purple with full maturity or exposure to light, as happens with many North American species. They are formed on long stolons that range from a few inches to about two feet long, with most plants having stolons on the longer side of that range. Tubers can remain dormant for very long periods. Bamberg (2010) found that tubers of many collections would store successfully at 41 degrees F (5 C) for eight years and recounted a story from a landowner who observed plants growing on resumption of irrigation after 39 years. While 39 years seems intuitively unlikely, the upper limit for dormancy in S. jamesii remains unknown and it will be a long wait for the report if they do turn out to last 39 years under controlled conditions!
Hale (2008) found that S. jamesii had high antioxidant activity and Kinder (2017) reported that wild collected tubers had twice the protein content of S. tuberosum, along with substantial increases in potassium, calcium, magnesium, and other minerals. These nutrient levels can probably be attributed primarily to the small size of the tubers. In the domesticated potato, smaller tubers have a greater proportion of nutrient-rich skin and cortex to flesh and this probably holds true in all potato species. Many wild species with similarly small tubers show similar increases in nutrition when compared to the domesticated potato. Along the same lines, potato skins look like a superfood in comparison to whole potatoes, since the interior of the potato is almost entirely carbohydrate.
S. jamesii was (and still is to a small extent) used for food by native Americans, likely cultivated to some degree, and possibly even improved. Louderback (2017) studied starch grains on ancient pottery and determined that this species was used as food in Utah as early as 8950 BC. If it were used consistently over such a long period, it would be surprising if it had not been improved through selection, even inadvertently. The primary pieces of evidence for its cultivation are (1) that populations are frequently found in close association with sites of previous human habitation; (2) that high levels of genetic diversity in some locations may indicate that plants were collected from other areas (Kinder 2017); and (3) that the tuber size and starch granule size of collections made near known archaeological sites are larger than those of collections not associated with such sites (Herzog 2018). Bamberg (2016) reported that that majority of the genetic diversity present in the USDA collection of this species could be found in one site at Mesa Verde, suggesting the possibility that many different genotypes were brought together there by man. According to Ortman (2018), although it later branched out to include other tuberous plants, the Tewa word sego refers specifically to Solanum jamesii, which must indicate that this was a plant of some importance prior to the introduction of other edible tubers that later were included under the same name. This evidence is sometimes combined to support the thesis that S. jamesii is the earliest domesticated potato. Domestication is most commonly held to mean that humans have made significant alterations to the plant through selection. If that was the case with S. jamesii, the differences between wild and relict cultivated populations are too small to easily substantiate. Overall, it seems like a stretch to describe this species as domesticated, but it makes for more attention grabbing headlines. That may change though, as there are efforts underway to expand the use of this potato in native communities, which could result in meaningful selection and improvement.
Whether or not it was domesticated, there are differences in edibility between S. jamesii and S. tuberosum. Of course, it is also true that edibility varies within S. tuberosum, with many examples on the wild fringes of that species that are not safe to eat nor palatable. You could easily come to incorrect conclusions about the edibility of this species by following the infrequent media coverage, which tends to describe it as not only edible, but more nutritious than S. tuberosum. We don’t have a lot of information about how this plant was eaten. Was it a regular part of the diet or a famine food, for example? Was it ever eaten fresh, or only in a processed form? In my experience, many plants of this species produce tubers with a sour or bitter aftertaste, which indicates a somewhat high glycoalkaloid content. There are also plants that produce tasty tubers with no sour aftertaste, but they are in the minority. Without knowing how the sour taste correlates with glycoalkaloid levels, I consider it unsafe to eat the sour tubers in amounts exceeding three ounces. The non-sour tubers are probably safe to eat, but that opinion is informed by observations made with other species, primarily S. tuberosum, and might not hold true with this species. As it stands, I think that you need to make palatable selections within this species, rather than indiscriminately consuming the tubers produced on all plants.
Jarvis (2008) predicts that this species will lose 91% of its present range by 2055 due to climate change, most likely entailing a critical loss of genetic diversity.
Resistances
This species can survive frosts down to 26 degrees F (-3.5 C) (Li 1977). Vega (1995) found that this species is less frost tolerant than domesticated potato. Johnson (1937) found that some tubers of this species were able to survive freezing for multiple days and Bamberg (2020) found that tubers could survive freezing down to 5 degrees F (-15 C) for as long as a week.
Pelletier (1999) found that Colorado potato beetles have increased adult and larval mortality and low egg production on this species.
This species is widely regarded as highly resistant to late blight, but I have observed the total range of responses, from rapid and complete defoliation to limited lesions to apparent immunity to the strain(s) of late blight present here. Seedlings, even of highly resistant lines, vary considerably in resistance. In 2021, a bad blight year here, I found that about 35% of a crop of 1200 seedlings succumbed completely to blight, another approximately 45% developed multiple lesions while remaining productive, and about 20% developed 0 to 2 small lesions of an indeterminate character (they could have been early blight or another fungal disease). Selection will be required to develop lines that are reliably resistant. At least one accession of this species carries a gene that confers resistance to the Chinese late blight strain (2013-18-306) that has cracked all S. demissum derived resistance genes (R1-R11) (Zheng 2020). I have also found S. jamesii to be extremely vulnerable to early blight, with plants of some accessions suffering severe damage under conditions in which most domesticated potatoes suffer very little.
| Condition | Type | Level of Resistance | Source |
|---|---|---|---|
| Alternaria solani (Early Blight) | Fungus | Not resistant | Jansky 2008 |
| Cold | Abiotic | Resistant | Bamberg 2020 |
| Drought | Abiotic | Resistant | Watanabe 2011 |
| Globodera pallida (Pale Cyst Nematode) | Invertebrate | Resistant | Castelli 2003 |
| Globodera pallida (Pale Cyst Nematode) | Invertebrate | Not resistant | Bachmann-Pfabe 2019 |
| Globodera rostochiensis (Potato Cyst/Golden Nematode) | Invertebrate | Resistant | Castelli 2003 |
| Leptinotarsa decemlineata (Colorado Potato Beetle) | Invertebrate | Somewhat resistant | Machida-Hirano 2015, Pelletier 1999 |
| Meloidogyne spp. (Root Knot Nematode) | Invertebrate | Somewhat resistant | Machida-Hirano 2015 |
| Myzus persicae (Green Peach Aphid) | Invertebrate | Not resistant to moderately resistant | Alvarez 2006 |
| Pectobacterium carotovorum (Blackleg/Soft Rot) | Bacteria | Not resistant | Chung 2011 |
| Phytophthora infestans (Late Blight) | Fungus | Resistant | Bhardwaj 2018 |
| Phytophthora infestans (Late Blight) | Fungus | Resistant | Bachmann-Pfabe 2019 |
| Potato Leaf Roll Virus (PLRV) | Virus | Somewhat resistant | Machida-Hirano 2015 |
| Potato Virus A (PVA) | Virus | Moderate | Webb 1961 |
| Potato Virus X (PVX) | Virus | Moderate | Webb 1961 |
| Potato Virus Y (PVY) | Virus | Somewhat resistant | Cai 2011 |
| Streptomyces scabiei (Scab) | Bacterium | Immune | Reddick 1939 |
| Synchytrium endobioticum (Wart) | Fungus | Somewhat resistant | Machida-Hirano 2015 |
Glykoalkaloid content
Gycoalkaloids in this species have been measured between 8.6 and 128 mg per 100 grams of fresh tuber (Johns 1990, Nzaramba 2009). Most of the published measurements fall into the lower part of the range. The generally accepted safety limit for potato glycoalkaloids is 20 mg / 100 g. People can usually perceive bitterness at about 10 mg / 100 g. Johns (1990) reported that the primary glycoalkaloid in this species is tomatine, unlike the domesticated potato, in which the primary glycoalkaloids are solanine and chaconine, but more recent work has reported that chaconine and solanine are present. If tomatine is the dominant glycoalkaloid, then the normal expectation that bitterness is proportional to glycoalkaloid concentration may not hold with this species. Johns (1986) reported that Aymara potato cultivators could not detect a significant flavor difference in tomatine solutions ranging from 40 to 80 mg / 100 ml, while they could easily detect differences in the same range of solanine and chaconine. According to several sources, this species was traditionally eaten with clay or processed in some fashion and used dried (White 1944, Moerman 1998) in order to eliminate some of the glycoalkaloids.
If we assume that 128mg/100g is the upper limit for this species (which is certainly not a safe assumption), then a 150 pound person could probably eat about three ounces without experiencing symptoms of glycoalkaloid toxicity. This is just an example and you should not consider it any kind of guidance for consuming this species. There are no named cultivars and thus no way to know how the glycoalkaloid levels might vary from one plant to another. It is possible that it is overly conservative to assume the same safe dosage for tomatine as for the more common potato glycoalkaloids solanine and chaconine. Green tomatoes, which are a fairly common food, may contain anywhere from 50mg/100g to 500mg/100g of tomatine. Although it hasn’t been studied in humans, this suggests that we may tolerate tomatine better than the more common potato glycoalkaloids.
Many people report eating this species without ill effect and I have done so myself, but there are clear indications that it contains a higher level of glycoalkaloids than domesticated potatoes. As with most potatoes that contain tomatine, I find that the tubers with (presumably) higher glycoalkaloids tend to taste more sour than bitter, although some plants produce tubers that are also moderately bitter. Overall, I think that S. jamesii is probably pretty safe, but we are working with a small amount of somewhat contradictory information, so it makes sense to proceed carefully until we know more. The reports that S. jamesii was traditionally eaten with clay are a warning sign, regardless of what laboratory results say.
I recommend the following procedure for taste testing S. jamesii tubers, although I cannot guarantee that even this process will result in safe levels of glycoalkaloids. Select some of the larger tubers that have not been exposed to sunlight. Pierce the tuber skin with a fork and then microwave for 60 seconds. Check to see if the flesh is soft and, if not, microwave for 30 more seconds. (Note that if you don’t pierce the skin, they may explode rather violently.) You can also taste them raw, which saves a step if you are not bothered by eating raw potatoes. Eat the whole tuber, skin and all. Put the tuber in your mouth and roll it around for a few seconds, tasting the skin, being alert to any sourness. Chew it slowly. Try to make it last a full minute, if possible. Pay particular attention to the skin and whether or not it seems sour now that you are chewing it. Otherwise, after you have chewed for a good minute, be alert to any bitter flavor toward the back of your tongue or the roof of your mouth. If you detect sourness or bitterness any any point in the process, you should consider tubers of that plant unsafe to eat in large amounts. If you get a bitter one, its aftertaste may linger for 20 minutes or more. If you have tasted a bitter tuber, I recommend waiting an hour before testing another plant, because you become desensitized to the bitterness. If you get through the whole process with nothing but normal potato flavors, congratulations, you have a plant that produces palatable tubers.
All of the above is focused entirely on the tubers. As with any other potato, the foliage, flowers, and berries likely contain higher concentrations of glycoalkaloids and should be considered toxic and dangerous to eat.
Images
Cultivation
Solanum jamesii grows in the high desert of the southwest United States and northern Mexico. It is adapted to dry conditions, growing in sandy soils, with hot days and cold nights. In the wild, S. jamesii usually sprouts during the rainy season in July and August (Kinder 2017). Plants flower and fruit from June to October (Spooner 2004). In cultivation, with irrigation, it can be started much earlier in the year, but I have found that flowering is poor here from an April sowing. While the plants emerge and grow well, they produce few flowers. In 2020, I planted in late June and the plants flowered more abundantly in our slightly warmer summer weather.
Tubers of this species have very long dormancy and that dormancy is not always easy to break. Tubers sometimes do not sprout the same year that they are planted. Planting into warm soil and keeping it well watered increases the probability of sprouting. I recommend mixing tubers into an equal amount of damp potting soil in a plastic bag and leaving it in a warm place. Plant the sprouted tubers. This way, you will achieve a much more even stand than you would just planting dormant tubers.
I have found S. jamesii seeds easy to germinate, using the standard conditions for S. tuberosum. Albino or chlorophyll deficient seedlings seem to be common in S. jamesii. The albinos will die as seedlings and the variegated types are inferior and not worth keeping. The USDA potato genebank has observed that germination of some accessions of this species is inhibited by gibberellic acid (Bamberg 1999).
Towill (1983) found that seeds of this species stored at 1 to 3 degrees C germinated at 38 to 94% after 15 years.
As with most wild potatoes, I recommend growing S. jamesii either in pots or in buried mesh bags to keep the stolons under control. The tubers are small and form on long stolons, so harvest is a frustrating experience at best without some kind of containment. It could easily spread and become difficult to eradicate. Liter pots or bags work well for this species, although shallower and wider is better than deep and narrow containers.
S. jamesii is a rather shy flowerer in this climate, which is unusual, since virtually every other wild potato flowers well here. Our spring and summer temperatures may be too cool for it. On average, only about 1 in 10 plants flower and those only briefly. Bamberg (1995) found that at least some accessions of S. jamesii flower better at high temperatures than under typical temperate growing conditions. Flowering was better when greenhouse temperatures exceeded 100 degrees F for several hours during the day. Pollen viability was also about the same regardless of temperature in several accessions, although markedly lower in others.
Trapero-Mozos (2018) determined that this species will tolerate a temperature of 40 C even without prior acclimatization to warm temperatures.
Accessions Evaluated
The following accessions were examined to prepare this profile. I have evaluated 35/183 accessions currently available from the US Potato Genebank. Late blight and early blight were categorized as Low (0 to 2 lesions per plant), Moderate (multiple lesions but productive plants), High (significant defoliation).
PI 275168
Colorado, Mesa Verde National Park, Navajo Canon, 1958.
Moderate germination (52%). Foliage blue green to green. Plants short to mid height, bushy. Some with blue axils. Low late blight. Low early blight.
PI 564052
Arizona, Apache County, Nelson Reservoir, 1992.
Low germination (22%). Some showing tuberization at first transplant. Green foliage. Mid to tall plants, bushy. Low late blight. Low early blight.
PI 564055
New Mexico, Catron County, Quemado, 1992.
Low and slow germination (8%). Green foliage. Tall plants. Some early flowering. Low late blight. Low early blight. High tuber counts, small to intermediate size, some palatable.
PI 585117
New Mexico, Cibola County, Near Grants, 1992.
Slow and low germination (20%). Foliage blue green. Plants short, bushy. Some blue axils. Low late blight. Low early blight.
PI 585119
Colorado, Mesa Verde Nat. Monument at juct. Spruce and Navajo Canyons, 1994.
Low germination (16%). Green foliage and some with large leaflets. Some showing chlorosis. Plants short to mid height. Unusual yellow dwarf with very short internodes. Low late blight. Low early blight.
PI 592397
New Mexico, Near Albuquerque, just East of Tajique, 1995.
Slow but moderate germination (44%). Foliage blue green, many with yellow leaf base. Plants mid height, bushy. Some early flowering. Some blue axils. Low late blight. Low early blight.
PI 592411
Arizona, Apache County, near Eagar, 1995.
Slow and low germination (16%). Green foliage. Mid-sized plants. Some early flowering. Low late blight. Low early blight. Low to intermediate tuber counts, intermediate size, mostly palatable.
PI 592416
Arizona, Apache County. Near Eagar, 1995. Large plants noted.
Irregular but moderate germination (52%). Foliage blue green to green. Plants mid height. Some blue stems, axils. Low late blight. Low early blight.
PI 595783
New Mexico, San Miguel Co, 1996. Large plants noted.
Irregular and low germination (12%). Foliage blue green. Plants mid height. Low late blight. Low early blight.
PI 603051
Utah, Garfield County. Escalante, 1997.
Low germination (22%). Foliage green, some chlorotic, some with large leaflets. Plants mid to tall. Low late blight. Low early blight.
PI 603052
Utah, Garfield County. Escalante, 1997.
Low germination (8%). Mostly blue green foliage. Bushy, mid-sized plants. Some blue axils. Moderate late blight. Low early blight.
PI 603054
Colorado, Garfield County. Escalante, 1997. Noted Anasazi site.
Very slow but moderate germination (36%). Foliage green to blue green, some chlorotic. Plants mid to tall. Some blue axils. Moderate late blight. Moderate early blight.
PI 603055
New Mexico, San Juan County. NE of Crownpoint. Chaco Culture National Historical Park, 1997.
Slow and low germination (24%). Many pale yellow and albino seedlings. Mostly blue green foliage. Short to tall. Some early flowering. Low late blight. Low early blight. Intermediate tuber counts, small to intermediate size, some palatable.
PI 603057
New Mexico, San Juan County. NE of Crownpoint. Chaco Culture National Historical Park, 1997. Moderate germination (36%).
PI 605357
New Mexico, San Miguel Co. N of Pecos, 1998.
Slow and low germination (18%). Unusual strap-like cotyledons and short internodes in some seedlings. Green to blue green foliage. Plants short to tall. Some early flowering. Low late blight. Low early blight.
PI 605366
New Mexico, Cibola Co. Near Grants, 1998.
Low germination (12%). Foliage blue green, some with broad leaflets. Plants short to mid. Some blue axils. Some early flowering. Low late blight. Low early blight.
PI 605367
New Mexico, Catron Co. Near Pie Town, 1998.
Slow and low germination (12%). Earliest to flower from seed in 2020. Mostly green foliage. Tall plants. Some with dark blue axils. Low late blight. Low early blight. Low to high tuber counts, small to large size, low palatability.
PI 605369
New Mexico, Torrance Co. Near Tajique, 1998.
Low and very slow germination (6%). Mostly green foliage. Mid-sized plants. Moderate late blight. Low early blight. High tuber counts, small size, some palatable.
PI 612450
Colorado, Mesa Verde National Park. Junction of Spruce and Navajo canyons, 1998.
Low and very slow germination (6%). Green foliage. Small plants. Low late blight. Low early blight. Intermediate tuber counts, small to intermediate size, some palatable.
PI 612455
Arizona, Coconino County, 1999. Large leaves noted.
Slow and low germination (14%). Foliage green. Plants mid height, bushy. Some early flowering. Low late blight. Low early blight.
PI 632324
Arizona, Navajo County. Sitgreaves National Forest, 2002. Abundant fruit noted.
Low germination (8%). Indications of early tuberization at first transplant. Mostly green foliage. Tall plants. Some early flowering. Low late blight. Low early blight. High tuber counts, small to intermediate size, low palatability.
PI 632326
Arizona, Navajo County. Sitgreaves National Forest, 2002. Large plants and abundant fruit noted.
Slow and low germination (12%). Indications of early tuberization at first transplant. Foliage green, some with yellow leaf base. Plants short to mid. Low late blight. Low early blight.
PI 634364
Arizona, Mohave County. Fredonia area, 2003. Large plants noted.
Low germination (24%). Foliage blue green, some with yellow leaf base. Plants mid height, bushy. Some early flowering. Low late blight. Low early blight.
PI 641941
Utah, East of Escalante in the Escalante River Gorge, 2005.
Early but low germination (16%). Some seedlings with unusually short internodes. Foliage green, some with broad leaflets. Plants mid height. Low late blight. Low early blight.
PI 663210
Colorado, Mesa Verde. Navajo Canyon, 2011.
Moderate germination (28%). Foliage blue green. Plants mid to tall. Some blue axils. Some early flowering. Low late blight. Low early blight.
PI 664006
Arizona, Apache National Forest. Big Lue Mountains, 2011. Large plants with fruit noted.
Slow and low germination (12%). Foliage blue green. Plants short, bushy. Some blue stems. Moderate late blight. Moderate early blight.
PI 664009
New Mexico, Gila National Forest. Mogollon Mountains, 2011. Large plants with fruit noted.
Moderate germination (32%). Foliage green. Plants mid to tall. Some early flowering. High late blight. Low early blight.
PI 669594
Arizona, Apache National Forest. Near Eagar, 2013. Large plants with fruit noted.
Early and moderate germination (50%). Some showing tuberization at first transplant. Foliage green. Plants mid to tall. Some blue axils. Some early flowering. Moderate late blight. Moderate early blight.
PI 673364
Arizona, Sitgreaves National Forest. Near Chevelon Creek, 2014. Large plants with fruit noted.
Slow but moderate germination (50%). Foliage blue green to green, some with broad leaflets. Plants mid to tall. Some blue stems or axils. Some early flowering. Moderate late blight. Low early blight.
PI 676014
Arizona, Coconino National Forest. Near Flagstaff, 2015. Large plants with fruit noted.
Early and moderate germination (56%). Some showing tuberization at first transplant. Some with short internodes. Foliage blue green to green. Plants mostly short and some rosette-like in form. Low late blight. Low early blight.
PI 679921
Arizona, Sitgreaves National Forest. W of Eagar, 2016. Large tubers noted.
Very slow germination (14%). Foliage green, some chlorotic. Plants tall. Some early flowering. Moderate late blight. Low early blight.
PI 686446
New Mexico, Santa Fe National Forest. Near Glorieta, 2017. Large plants noted.
Slow and low germination (14%). Mostly green foliage. Mid-sized plants. Moderate late blight. Low early blight. High tuber counts, small to medium size, low palatability.
PI 689409
Arizona, Navajo County, 2018.
Moderate germination. Foliage green. Plants mid to tall. Low late blight. Moderate early blight.
PI 689422
New Mexico, Catron Gila Cliff Dwellings. Gila National Forest, 2018.
Moderate germination. Foliage green. Plants mid to tall. Low late blight. Low early blight.
PI 689425
New Mexico, Pinos Altos. Gila National Forest, 2018. Large plants and large tubers noted.
Requested, not received.
Breeding
As is true of most diploid potato species, S. jamesii is an outbreeder. More than one clone is required to produce seed. Most academic work is focused on crossing S. jamesii traits into domesticated potatoes. This turns out to be quite difficult. While S. jamesii will likely never be a practical economic crop, it may be possible to improve it for subsistence use by breeding within species. There is some variation in phenotype, including quite a bit of variation in size of the aerial plant, tuber size and number, and flowering duration. With a moderate amount of selection, I would not be surprised if a more reliably edible and more productive crop could be produced.
Crosses with S. tuberosum
There is a lot of interest in crossing S. jamesii with domesticated potatoes in order to introduce some of its interesting features like long dormancy to domesticated lines. Unfortunately, it is very difficult to make crosses between this 1EBN species and either 2EBN or 4EBN domesticated potatoes, perhaps impossible. The most direct route would be to cross S. jamesii to a diploid domesticated potato, relying upon S. jamesii to produce some unreduced 2x(2EBN) gametes. Due to the difference in EBN, the expected result of such a cross would be a triploid with two copies of the S. jamesii genome and one copy of the S. tuberosum genome. As far as I know, such crosses have been rarely reported and the progeny have not been proven to be the result of successful crosses. Even if such a cross were successful, the triploid progeny would likely be difficult to use in further breeding, although could possibly be doubled to a 4EBN hexaploid that could, hypothetically, be crossed with tetraploid S. tuberosum. I don’t know that any of this is impossible, but if it were easy, it would already have been done many times.
Another possible route would be to attempt to cross S. jamesii with a 2EBN tetraploid species like S. acaule or S. stoloniferum, again relying upon S. jamesii to produce unreduced gametes. This would yield 2EBN tetraploid progeny with 2 copies of the S. jamesii genome and two copies from the other parent. The progeny could then be crossed to diploid domesticated potatoes, resulting in a triploid, or possibly be doubled to a hexaploid for direct crosses with tetraploid S. tuberosum. As with the previous scenario, there is no information indicating that this has been accomplished.
The only approach for crossing S. jamesii that is well documented is the use of S. verrucosum, a more closely related 2EBN diploid species. S. verrucosum has shown some ability to cross successfully with S. jamesii, although the rate of success is low. Despite the low rate, the progeny of such crosses are 2EBN diploids, providing the ability to cross directly to domesticated diploid potatoes, or to tetraploids through unreduced gametes. (The results of this cross are quite interesting, as they do not agree with the predicted outcome based on ploidy and EBN, which would be a triploid with two copies of the S. jamesii genome and one of the S. verrucosum genome. There appears to be something special about the way that S. verrucosum behaves in crosses.)
Livermore (1940) attempted to make reciprocal crosses between tetraploid S. tuberosum and chromosome doubled S. jamesii without success. This is consistent with what we know of S. jamesii. It is a diploid species with 1EBN. After chromosome doubling, it would behave as a tetraploid with 2EBN. Because S. tuberosum is tetraploid and 4 EBN, the cross is not compatible even after doubling.
| Female | Male | Berry Set |
Seed Set | Germ | Ploidy | Source |
|---|---|---|---|---|---|---|
| S. jamesii | S. tuberosum (4x) |
None | None | Jackson (1999) | ||
| S. tuberosum (4x) |
S. jamesii | None | None | Jackson (1999) |
Crosses with other species
The easiest crosses to make with S. jamesii will be those with other 1EBN diploids. The problem is that most of those species have similar deficits to S. jamesii, namely small tubers and long stolons. S. ehrenbergii and S. cardiophyllum (collectively known as cimatli), both have been through some light domestication and there are types with larger tubers. Although they would appear to be evolutionarily close matches, crosses between S. jamesii and S. cardiophyllum or S. ehrenbergii appear to be quite difficult, perhaps impossible.
Matsubayashi (1977) examined chromosome pairing in hybrids and determined that S. x sambucinum and S. jamesii were highly compatible and likely closely related. They found irregular chromosome pairing and reduced pollen fertility in crosses with S. bulbocastanum.
Watanabe (1991) found that 7.8% of varieties of this species produced 2n pollen and Jackson (1999) found 1-18%, which would be effectively tetraploid and 2EBN.
| Female | Male | Berry Set |
Seed Set | Germ | Ploidy | Source |
|---|---|---|---|---|---|---|
| S. jamesii |
S. bulbocastanum |
Yes | Yes | 2x | Matsubayashi 1977 | |
| S. jamesii | S. commersonii | Yes | Yes | Reddick 1939 | ||
| S. jamesii |
S. polyadenium |
Yes | Yes | Magoon 1958 | ||
| S. polyadenium |
S. jamesii |
Yes | Yes | Magoon 1958 | ||
| S. x sambucinum |
S. jamesii |
Yes | Yes | 2x | Matsubayashi 1977 |
References
Solanum jamesii at Solanaceae Source
Solanum jamesii at GRIN Taxonomy

































Regarding toxicity of S. Jamesii., it grows in thisarea. The native people, and others, reccomend eating only newer immature tubers. Just put the old one back. It is much stronger.
Thanks Robert, that’s interesting information. That may help to account for the conflicting information about edibility with this species.
I brought tubers from an archaeological site in Cortez, CO, to southern Vermont ten years ago. I have found that they are very tolerant of the acidic, moist soul in my woodland (mixed hardwoods) location and overwinter well. Although in the past they have run on the typical Southwestern time schedule of sprouting in mid-July, this year they sprouted in mid-June and produced prolifically. I became acquainted with S. jamesii through Dr. David Kinder of Ohio Northern University, when we met at Mesa Verde about 15 years ago. A volunteer in archaeology in Southwestern CO, I have “played with” S. jamesii since that time. I’ve tried to inquire of various Anishanabe and Abenaki people, if they are familiar with this plant as a food item, possibly traded up from the Southwest, but have had no responses. Having continued to correspond with Dr. Kinder and become very interested in some of the nutritional studies of this tuber, I plan to cultivate them for my own consumption in my little woodland garden. Your long article is very informative in areas with which I was not previously familiar, and I am very much interested in what you have said.