Blog, genetics, potato (Solanum tuberosum)
Potato Cytoplasm Types
If it has been a long time since high school biology and you are rusty on the concept of cytoplasm, you can be forgiven. It’s not something that most of us think about very often, but it is important to potato breeding, so let’s review.
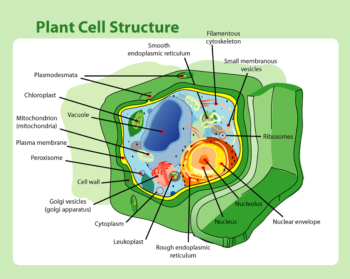 Cytoplasm is everything that is inside a cell but outside the nucleus. The nucleus primarily contains DNA, in the form of chromosomes, that determines nearly all the traits of the organism. The cytoplasm contains a diverse array of cellular machinery in a fluid medium. Two pieces of that cellular machinery, the mitochondria and the chloroplasts, are very important in plant breeding. Chloroplasts produce glucose through photosynthesis and mitochondria convert glucose into ATP, which the cell uses as an energy source. Both chloroplasts and mitochondria contain their own genetic code and it is inherited separately from the DNA in the nucleus. They have separate DNA from the nucleus most probably because they were once separate organisms. Chloroplasts and mitochondria are generally accepted to once have been other unicellular life forms that became incorporated into the cell at the end of some long mutualistic relationship. The details aren’t important, but if you think of the mitochondria and chloroplasts as two separate organisms, this will all make more sense. The genetic code of the mitochondria and the chloroplasts is known collectively as the “cytoplasmic genome,” presumably because people got tired of typing “mitochondrial genome and chloroplast genome.” We shorten that even moreso when talking about plant breeding to simply “cytoplasm.”
Cytoplasm is everything that is inside a cell but outside the nucleus. The nucleus primarily contains DNA, in the form of chromosomes, that determines nearly all the traits of the organism. The cytoplasm contains a diverse array of cellular machinery in a fluid medium. Two pieces of that cellular machinery, the mitochondria and the chloroplasts, are very important in plant breeding. Chloroplasts produce glucose through photosynthesis and mitochondria convert glucose into ATP, which the cell uses as an energy source. Both chloroplasts and mitochondria contain their own genetic code and it is inherited separately from the DNA in the nucleus. They have separate DNA from the nucleus most probably because they were once separate organisms. Chloroplasts and mitochondria are generally accepted to once have been other unicellular life forms that became incorporated into the cell at the end of some long mutualistic relationship. The details aren’t important, but if you think of the mitochondria and chloroplasts as two separate organisms, this will all make more sense. The genetic code of the mitochondria and the chloroplasts is known collectively as the “cytoplasmic genome,” presumably because people got tired of typing “mitochondrial genome and chloroplast genome.” We shorten that even moreso when talking about plant breeding to simply “cytoplasm.”
(Although not important in the context of this post, there are additional organelles in the cytoplasm that contain their own genetic code. They are known as leucoplasts as a group. In potato, one important leucoplast is the amyloplast, which produces and stores starch. Just as with the mitochondria and chloroplasts, they are thought to have originated as separate organisms, probably cyanobacteria. These also make up part of the cytoplasmic genome, but they are less studied. Viruses inhabiting the cell may also be considered part of the cytoplasmic genome. So, when we talk about the cytoplasmic genome, we’re usually simplifying a lot.)
During sexual reproduction, the DNA in the nucleus gets mixed and matched. Chromosomes from the male and female intermingle and combine, so the resulting progeny has a mix of genes from both parents. The situation is completely different for mitochondria and chloroplasts. When the potato flower forms an egg cell, it is derived from an existing cell and inherits its set of mitochondria and chloroplasts from the potato plant. Later, when that egg merges with a sperm, it keeps only the sperm’s DNA. It retains the cytoplasm that it originally received. The important thing to understand here is that the cytoplasm always inherited from the mother. They are genetically identical to the mother’s copies. Nuclear DNA is a mix of mom and dad, but cytoplasmic DNA is always mom.
Why does this matter? Because potato varieties each carry their own unique mitochondria and chloroplasts and these sometimes confer important traits.
Potato cytoplasm has been classified under a number of different types, defined primarily by the population of potatoes from which that cytoplasm originated. As with many aspects of potato research, classification of cytoplasm types has evolved over time and several different systems have been used. The most modern system (Hosaka 2012) incorporates both chloroplasts and mitochondria into a single classification system. The types are M (Mother), A (Andigena), P (Phureja), W (Wild), D (Demissum), and T (Tuberosum). Separate classification systems also exist for chloroplasts and mitochondria. Chloroplasts are classified as C, A, S, W, and T. Mitochondria are classified as α (alpha), β (beta), γ (gamma), and the much less common d (delta) and e (epsilon). You need to be aware of both systems to read the literature and some parts, particularly the mitochondrial types, are still commonly used. There are also some much rarer types that don’t fit into these groups. One system of classification divides cytoplasm into 164 haplotypes, a level of resolution that is probably not useful to breeders. The following chart includes types defined by both of the systems that are commonly used in research papers. The bolded rows are the most modern system.
| Type | Species and Varieties | Traits | Frequency |
| M (C) |
Occasionally found in Andean cultivated potatoes but more commonly in the wild ancestors from which they were derived. Previously described as C (Canasense) type. Species: S. acaule, S. acroscopicum, S. agrimoniifolium, S. candolleanum, S. colombianum, S. dolichocremastrum, S. flahaultii, S. iopetalum, S. juzepczukii, S. lignicaule, S. longiconicum, S. medians, S. multiinterruptum, S. raphanifolium |
|
Very rare in modern potatoes; when it is present, it probably indicates breeding with S. acaule. Found in only 0.3% of European potatoes (Sanetomo 2015) and 0.2% of Japanese potatoes (Hosaka 2012). Relatively common in tetraploid andigena type potatoes in southern Peru, Bolivia, and Argentina. |
| A (A/e) |
Species: Most 4x and some 2x S. tuberosum subsp. andigenum, S. maglia Varieties: Brodick2, Fifty-fold2, Lumper1, Maris Piper2, Myatt’s Ashleaf1, Pink Fir Apple1, Shelagh2, Skirza2, Stormont Enterprise2 |
Uncommon in modern potatoes since andigena potatoes are more often used as a pollen parent. Found in only 0.7% of European potatoes (Sanetomo 2015) and 1.2% of Japanese potatoes (Hosaka 2012). The most common cytoplasm type in tetraploid andigena potatoes, with the possible exception of Bolivia, where C type cytoplasm is also common. |
|
| P (S, S/e) |
Previously described as S (Stenotomum) type. Species: 2x S. tuberosum subsp. andigenum (Phureja and Stenotomum type potatoes), some 4x S. t. subsp. andigenum, S. ajanhuiri and S. curtilobum, some S. candolleanum. Varieties: Inca-no-mezame, Saikai 35 |
Uncommon in modern potatoes since phureja potatoes are more often used as a pollen parent. The exception is Japan, where Phureja has been used as a female parent in several popular breeding lines. Absent in European potatoes (Sanetomo 2015) but found in 6.4% of Japanese potatoes (Hosaka 2012). Predominant in diploid andigena throughout the Andes. Occasionally found in tetraploid andigena as well. |
|
| W |
Chilean cultivated potatoes and most wild potatoes. Subtypes, located at the bottom of the table, are defined based on a mitochondrial marker. Rarely found in Andean domesticated potatoes in the southern Andes. Species: S. boliviense, S. brevicaule, S. chacoense, S. immite, S. pinnatisectum, S. sogarandinum, S. stoloniferum, S. vernei, more listed below |
Common in modern potatoes. Found in 12.2% of European potatoes (Sanetomo 2015) and 2.3% of Japanese potatoes (Hosaka 2012). Rare Peru northward, becoming more common in Bolivia and south. |
|
| D (W/α) |
Species: S. demissum and modern potatoes bred from it |
|
Common in modern potatoes, very common in German varieties. Found in 27.4% of European potatoes (Sanetomo 2015) and 17.8% of Japanese potatoes (Hosaka 2012). |
| T (T/β) |
Species: S. berthaultii, S. neorossii, S. tuberosum subsp. chilotanum, most modern potatoes, occasionally found in Andean potatoes in the southern Andes, although it isn’t totally clear if they should be considered andigena if they have type T cytoplasm. Varieties: All Blue (Congo)2, Beauty of Hebron, Desiree, Early Rose, Early Ohio, Estima2, Garnet Chili, Green Mountain, Irish Cobbler, Katahdin, Kennebec, Kerr’s Pink2, King Edward2, Norland, Russet Burbank, Shepody2, Yam |
|
Very common in modern potatoes, 60% or more are T type. Found in 59.4% of European potatoes (Sanetomo 2015) and 72.1% of Japanese potatoes (Hosaka 2012). Rare in Argentina and common in Chile. Not present in the other Andean countries other than through introduced modern potatoes. |
|
W/γ
|
Species: S. stoloniferum, S. chacoense, S. pampasense, S. pinnatisectum, S, vernei, and modern potatoes bred from those species |
|
Common in modern potatoes |
| W/d | Uncommon in modern potatoes | ||
|
W/β
|
|
Uncommon in modern potatoes | |
|
W/αβ
|
Species: Some S. chacoense, S. spegazinni Varieties: Rita |
Rare | |
| 1 Provan (1999) 2 Powell (1993) | |||
Types A and P are variants of M and types D and T are variants of W. W is particularly diverse, as it covers many wild potato species.
The most important trait conferred by cytoplasm is male sterility. Male sterility occurs for different reasons, but amounts to the same thing in practice: male sterile varieties can only be used as female parents. Since cytoplasm is inherited from the mother, there is no way to reverse male sterility. All of the progeny of a male sterile variety will also be male sterile. D and W/γ cytoplasm types are strongly male sterile, while T type cytoplasm has poor male fertility. These three types comprise more than 90% of modern potato varieties.
The cytoplasmic genome also affects other traits. Late blight foliage resistance, starch content, yield, and flowering are affected by some types of cytoplasm. Unfortunately, the types of cytoplasm that confer these benefits also often produce male sterility.
Potato cytoplasm has also played an important role in unraveling the history of modern potato breeding. The earliest examples of European potatoes have A type cytoplasm, indicating that they were introduced from the Andes. These varieties were dropped almost entirely following the introduction of late blight to Europe and replaced by varieties that have T type cytoplasm, presumably of Chilean origin. Varieties with T type cytoplasm now comprise the majority of modern potato varieties. In 1908, it was discovered that the wild potato species Solanum demissum had resistance to late blight. Breeding with S. demissum began in earnest in Germany and introduced D type cytoplasm to modern potatoes. Later efforts at breeding with other wild species introduced W type cytoplasm to the modern potato gene pool. T, D, and W types are all fairly common in modern potatoes, while M, A, and P are unusual. (Of course, these types are very common in Andean potatoes.)
If you have gotten this far, you might be wondering how this information is useful. One way it can help to direct your breeding is by avoiding male sterility in potato crosses. Male sterility isn’t all bad; it allows you to produce a lot of cross pollinated seed without having to segregate varieties or emasculate the flowers. On the other hand, it limits the kind of crosses that you can perform. Varieties with full fertility can be used more flexibly. By avoiding parents with T, D, and W cytoplasm, you greatly increase the chance that new varieties will be fully fertile.
Another use is perhaps less practical, more a matter of philosophy. Many people are working with potatoes that have the common cytoplasm types. If you are an amateur or specialty breeder, why not try something different? The M, A, and P type cytoplasms of Andean potatoes are not well represented in modern potatoes. There are a variety of reasons for this, but the main one is that they simply haven’t been used much. Tom Wagner has often mentioned that he likes to work with the old Irish variety Lumpers because it is one of the last few European holdouts with A type cytoplasm (and it has a fairly rare haplotype of that cytoplasm). I can’t point you to a single reason why the A type cytoplasm from Lumpers is a better choice than any other potato, but my instinct as a plant breeder is to preserve and expand diversity whenever possible. The limited number of cytoplasm types present in modern varieties is often referred to as a genetic bottleneck. Genetic bottlenecks create an environment where pests and diseases can suddenly get the upper hand. For this reason, if no other, I think it is a particularly good idea for small breeders to range far afield. By including more Andean varieties and wild species in your breeding projects, you can diversify the types of cytoplasm that are included in your population.

So if you start with a known cytoplasm type and track the female lineage you can always know the cytoplasm type. But if you don’t know the female cytoplasm type how can that be determined? is there some way for a hobbyist to determine cytoplasm type or does it require more expensive testing?
Cytoplasm types are determined by PCR, which is probably out of reach of most amateurs, although undergrads do it all the time, so it is certainly possible if you are determined. It requires some equipment, which will probably set you back around $1000 used. I’m not aware of anyone who offers cytoplasm ID as a service, but some universities might. Otherwise, you would want to start with varieties for which the cytoplasm type has been published and track your pedigrees. Virtually every commercial potato is T type and I have linked in the article to some genebank accessions and other varieties that have been identified.